A Scientist Speaks: Senate Bill 277 in California

Distinguished Members of the California Senate Committee, The argument of forcing a parent to vaccinate their child in the name of the “greater good argument” is flawed both scientifically and ethically. First, all drugs are associated with some risks of adverse reactions. Because vaccines represent a special category of drugs which are by and large given to healthy individuals, and for prophylaxis against diseases to which an individual may never be exposed, the margin of tolerance for side effects is very narrow (in fact, the U.S. Food and Drug Administration (FDA) concurs with this point...
) and careful assessment of risks versus benefits essential in deciding whether one should be vaccinated or not. Removing the parental rights to exemptions to childhood vaccinations will put vulnerable but otherwise healthy individuals at risk of serious adverse reactions to vaccinations.
Such an outcome should be of concern since serious adverse reactions following routine vaccinations in children, including deaths, permanent neurological damage and disabling autoimmune and/or inflammatory conditions have been clearly described in the scientific literature
. Notably, cases of seizure attacks and deaths occurring as a result of routine vaccinations have occurred even in children and individuals without any relevant prior medical history
and in some cases a direct causal link was established between vaccination and the serious adverse reactions
. Please consider carefully whether you wish to be responsible for any of the above mentioned potential outcomes should you facilitate this legislation to come to pass.
Second, medical ethics demand that vaccination should be carried out with the participant’s full and informed consent. This necessitates an objective disclosure of the known or foreseeable vaccination benefits and risks. The way in which pediatric vaccines are often promoted by various health authorities indicates that such disclosure is rarely given from the basis of best available knowledge but rather, largely unproven and/or untenable assumptions on both, vaccine safety and effectiveness. I shall herein elaborate on these arguments.
Is Vaccine Safety Evidence “Rock Solid”?
In spite of the widespread notion that vaccines are largely safe and serious adverse complications are extremely rare, a close scrutiny of the scientific literature does not support this view
. Indeed, it is often assumed that vaccines face a tougher safety standard than most pharmaceutical products. However, according to the U.S. Food and Drug Administration (FDA) transcript of the 2002 Worksop on non-clinical safety evaluation of preventative vaccines: recent advances and regulatory considerations
:
“Historically, the non-clinical safety assessment for preventive vaccines has often not included toxicity studies in animal models. This is because vaccines have not been viewed as inherently toxic”
This is a startling admission from an Agency which according to its own mission statement is ”responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs” [17]
. Essentially, what the FDA workshop
revealed is that not only are vaccines not adequately evaluated for toxicity but also, that the reason for such an oversight rested on a belief rather than scientific evidence. Moreover, it is mind-boggling that inadequately tested products on whose safety FDA “places significant emphasis” are actually licensed by the same Agency for mass use.
Furthermore, the erroneous assumptions of safety, in the absence of actual experimental data, are not only dangerous but have historically hampered serious scrutiny of potential vaccine harms. For example, in responding to numerous criticisms of their study Unexplained cases of sudden infant death shortly after hexavalent vaccination
Zinka et al. (2006) noted
:
“(ad 6) The main problem is that vaccination specialists have failed for decades to establish any tests or other criteria to find out if adverse events are linked to vaccinations or not. To our knowledge they did not even try hard—why?!”
“(1) A precise description of the mechanism leading to serious adverse events after hexavalent vaccination is not the task of forensic pathology. This would be the job of vaccination specialists, and actually this job should have been done before phase 1 and phase 2 studies in order to get valid data on the drug safety.”
Similarly, in 2006, Ottaviani et al.
in reporting a case of a 3-month-old female infant who died shortly after being given a hexavalent vaccination noted that:
“This case offers a unique insight into the possible role of hexavalent vaccine in triggering a lethal outcome in a vulnerable baby. Any case of sudden unexpected death occurring perinatally and in infancy, especially soon after a vaccination, should always undergo a full necropsy study according to our guidelines…The identification of a possible pathological basis of reflexogenic mechanisms in sudden, unexpected infant death necessarily requires examination of the brainstem nuclei and of the cardiac conduction system on serial sections.”
The senior author of this study, Professor Luigi Matturri is a member of the European Medicines Agency (EMEA) Pathologists Panel for evaluation of SUD (sudden unexpected death) cases reported for hexavalent vaccines. Although a review by EMEA cited in the study concluded that the causes of death following hexavalent vaccination remained unexplained, the following was also emphasized:
“However, to the best of our knowledge, during the mentioned post-mortem investigations, little, if any, attention was paid to examination of the brainstem and the cardiac conduction systems on serial sections, nor was the possibility of a triggering role of the vaccine in the lethal outcome considered”
[4].
It is thus obvious that the real reasons why causality is rarely established by scientific investigations of vaccine-related serious adverse reactions are:
- it is assumed that vaccines cannot cause such reactions (as implied by the FDA workshop) and;
- studies are not designed to detect them
[19]
We have also noted that too often clinical trials of new vaccines conducted by drug companies are fast tracked to licensure but
1) fail to use inactive placebos as controls;
2) include too few children in the age group that will be targeted for universal use;
3) have inadequate periods of time for follow up of safety and effectiveness;
4) only study healthy children without personal or family histories of vaccine reactions, autoimmunity, allergy, neurological disease or concurrent illness (although children with these medical histories are specifically targeted for vaccination post-licensure with very few medical contraindications listed to guide physicians);
5) fail to study large numbers of children given the experimental vaccine simultaneously with all other vaccines routinely administered simultaneously to children in that age group;
6) dismiss serious health problems, injuries and deaths occurring during the trial as not related to the experimental vaccine without adequate research evidence-based support;
7) use questionable surrogate endpoints to demonstrate vaccine effectiveness; and
8) lack adequate post-licensure follow-up
.
The pushing of poorly tested drugs on most vulnerable populations (i.e., infants and children) can hardly be viewed as ethical. Unfortunately it is a frequent occurrence in medical practice when it comes to vaccination. To illustrate the consequences of such practices, in 2010 in Australia, there were a large numbers of serious adverse reactions from seasonal influenza vaccines routinely administered to children.
Subsequently, vaccination with certain influenza vaccines has been suspended in children under five years of age. In a series of Rapid Responses addressing this issue, published in British Medical Journal, titled “Adverse events following influenza vaccination in Australia-should we be surprised?” Peter Collignon (Director of Infectious Diseases & Microbiology at Australian National University) and colleagues from the Cochrane Collaboration review panel concluded
::
Collignon et al.
:
“Unlike most drugs, vaccines are used on a population basis triggered by public health policy. As such, evidence of their safety and efficacy needs to be extraordinarily rigorous and evaluation methods and data should be open to independent scrutiny. We need much better and larger studies on both safety and efficacy before we roll out influenza vaccine programs to all populations, especially to children who appear to have much higher rates of adverse reactions.
There is poor evidence on how well influenza vaccines prevent any influenza complications in children and other age groups. There is good evidence that influenza vaccines study reports cherry pick results and achieve spurious notoriety. Exposing human beings to uncertain effects is a risky business”
Collignon et al.
:
Vaccine policies must ensure they are doing more good than harm. Vaccine must cause far fewer serious adverse events compared to what the disease would have caused in the vaccine’s absence. Evidence suggests this is not the case with influenza. In Australia in 2009, during winter when young children (0-4 years) were first hit with the new H1N1 strain, the admission rate for influenza was 57 per 100,000 (8).
In the US, CDC says that influenza results in hospitalization for approximately 20 per 100,000 children aged 2 to 5 years (9), but vaccine-induced febrile convulsions resulting in hospitalization in US young children, likely occurred at a rate of 114 per 100,000 children vaccinated . According to the FDA, a “serious adverse event” is defined as hospitalization that results from a vaccine adverse event (10). Thus vaccinating young children without risk factors likely caused more serious adverse events than disease from the new “pandemic” itself. There is poor safety data available for other serious adverse events that might occur in young children in addition to febrile seizures (11).
Evidence from systematic reviews show evidence of data suppression of vaccine-associated harms to small children by some pharmaceutical companies (12). Other reports suggest that influenza vaccines put children at higher risk of future influenza infections compared to acquiring natural infection (original antigenic sin) (13). In older children, unexpected adverse events such as narcolepsy have been reported from at least 12 countries (14). In Canada previous immunisation with seasonal influenza vaccine doubled your risk of being infected with “swine flu” (15).
That the influenza vaccine is not an isolated case of poor scrutiny is evident from other literature on vaccines. Indeed, there is a growing number of reports of research misconduct, biased reporting, conflicts of interest, and outright fraudulent activity by pharmaceutical companies who produce the ever growing list of vaccines, bringing into question the accuracy of the vaccine manufacturers claims of safety and efficacy.
For example Merck & Co., Inc., the pharmaceutical company who produces the MMR (measles, mumps, and rubella) vaccine is currently accused in the U.S. of fraudulently lying about the efficacy of its mumps vaccine for the purpose of continuing to secure governmental contracts worth $ millions. In 2012, two former Merck virologists, a group of doctors, and direct payers filed two whistleblower law suits in the Pennsylvania federal court. Merck’s attorneys were unsuccessful in their attempts to block the case from going to trial with U.S. Federal District Judge C. Darnell Jones II, recently clearing the case for trial.
Judge Jones ruled the whistleblowers and direct purchasers produced enough evidence to establish that false statements could have helped give Merck a monopoly. A recent article from Pharma-based website fierce vaccines states
:
Merck has been the sole manufacturer with an FDA license to produce mumps vaccine since 1967, the news service points out, and the company has long touted a 95% efficacy rate for the shot. The drugmaker brought in $621 million on mumps vaccine sales last year, between its MMR2 vaccine and ProQuad, a pediatric combo jab.
But rather than using the “gold standard” approach and testing the vaccine against a wild-type mumps virus, Merck tested it against the attenuated virus strain that had created the vaccine in the 1960s–likely overstating the vaccine’s effectiveness, the whistleblowers claim, according to the judge’s memorandum.
And if Merck “fraudulently misled the government and omitted, concealed, and adulterated material information regarding the efficacy of its mumps vaccine” in violation of the False Claims Act, as they allege, it may have discouraged competition.
“This decision brings us one step closer to shining a light on Merck’s deceptive business practices so that new and more effective vaccines will ultimately be developed in the future,” Robins Kaplan Miller & Ciresi lawyer Kellie Lerner said in a statement.
Furthermore, with regard to the studies which allegedly demonstrably show no link between autism and vaccines, it has to be emphasized that once such studies undergo proper expert scrutiny, the “evidence” against the link becomes rather flimsy. In reviewing the published literature on measles-mumps-rubella (MMR) vaccine (139 studies), the respected Cochrane Collaboration review panel concluded that, “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate”
.
Moreover, none of the 31 studies that were included in the review met the Cochrane Collaboration’s methodological criteria. More specifically, referring to the 2001 Fombonne and Chakrabarti study
which was widely regarded by medical health authorities as most persuasive in disproving the link between the MMR vaccine and autism, the Cochrane Collaboration commented the following: “The number and possible impact of biases in this study was so high that interpretation of the results is impossible”
.
Although the Cochrane Review on the safety of MMR concluded that there was no credible link between MMR vaccination and autism and Crohn’s disease, as pointed out earlier, the majority of the studies included in the evaluation were methodologically inadequate. The question thus is what “credible” evidence can be derived from inadequate and/or methodologically flawed studies?
It important to note that even those in the scientific community who are strong proponents of vaccinations have come to question the scientific legitimacy of “one-size fits all” vaccination practices
. For example, Poland (Editor in Chief of the journal Vaccine and co-author of “The age-old struggle against the antivaccinationists”
) and colleagues rightly ask whether “with the advances coming from the new biology of the 21st Century”, it is time to consider “how might new genetic and molecular biology information inform vaccinology practices of the future?”
. In light of this question Poland et al. conclude that “one-size fits all” approach for all vaccines and all persons should be abandoned.
According to Poland, this conclusion applies to both vaccine efficacy, as well as safety
. Regarding the latter, the widely held view that serious vaccine-related adverse reactions are rare needs revision, as current worldwide vaccination policies indeed operate on “one-size fits all” assumption. This assumption persists despite the fact that historically, vaccine trials have routinely excluded vulnerable individuals with a variety of pre-existing conditions (i.e., premature birth, personal or family history of developmental delay or neurologic disorders including epilepsy/seizures, hypersensitivity to vaccine constituents etc.
).
Because of such selection bias, the occurrence of serious adverse reactions resulting from vaccinations may be considerably underestimated. As mentioned previously, such an outcome should be of concern in view of documented evidence of permanent neurodevelopmental disabilities and deaths following vaccination in children with underlying genetic and other susceptibilities
. Poland et al.’s current data may thus have far broader implications for understanding vaccines, not only in terms of efficacy and the desired immune response, but also in terms of safety. Indeed, vulnerable populations will neither have the same antibody response nor the same level of tolerance to serious adverse reactions as non-vulnerable populations
.
Under-appreciated risks associated with vaccines: aluminium adjuvants and repeated over-stimulation of the immune system by multiple closely-spaced vaccinations
The safety issue of aluminum adjuvants in vaccines has likewise been overlooked by the regulators (for more than 90 years since these compounds have been in use) as shown by the following statement made in 2005 by the World Health Organization (WHO) special Committee on the Safety of Vaccines
“The Committee considered the safety of adjuvants used in vaccines. This hitherto neglected subject is becoming increasingly important given modern advances in vaccine development and manufacture.”
What should be obvious from the above is that the current presumed evidence of safety of aluminum adjuvants has not been established as widely thought, rather, what we have here is a clear evidence of negligence regarding this subject by the world’s highest heath authority.
On the other hand, research evidence from independent sources (i.e., not sponsored by the vaccine manufacturers) shows that aluminum in vaccine-relevant exposures is toxic to humans and animals
, although such opinions appear to be highly regarded, they contradict basic toxicological principles. For example, it should be obvious that the route of exposure which bypasses the protective barriers of the gastrointestinal tract and/or the skin will require a much lesser dose to produce a toxic outcome
. In the case of aluminum, research clearly shows that only ~0.25 % of dietary aluminum is absorbed into systemic circulation
, while aluminum from vaccines may be absorbed at nearly 100% efficiency
.
Macrophagic myofasciitis (MMF) is one of the post-vaccinal conditions that has been solidly linked to the long-term persistence of vaccine derived-aluminum adjuvants (up to 8-10 years following vaccination;
. The pathological significance of the MMF lesion has long been ill-understood because of the lack of an obvious link between persistence of aluminum agglomerates in macrophages at sites of previous vaccination and delayed onset of systemic and neurological manifestations. However, recent experiments in animal models have revealed that injected nano-aluminum adjuvant particles have a unique capacity to travel to distant organs including the spleen and the brain
and incite deleterious immuno-inflammatory responses in neural tissues
, Moreover, the Trojan horse-mechanism by which aluminium enters the brain, results in its slow accumulation and is likely responsible for cognitive impairments associated with administration of aluminium-containing vaccines
.
The bioaccumulation of aluminium in the brain appears to occur at a very low rate in normal conditions, thus potentially explaining the presumably good overall tolerance of this adjuvant despite its strong neurotoxic potential. Nonetheless, according to Khan et al.
, continuously increasing doses of the poorly biodegradable aluminium adjuvant may become insidiously unsafe, especially in cases of repetitive closely-spaced vaccinations and immature/altered blood brain barrier.
In this context, the latest research by Lujan et al.
who described a severe neurodegenerative syndrome in commercial sheep, linked to the repetitive inoculation of aluminium-containing vaccines, is noteworthy. In particular, the “sheep syndrome” is similar to some human diseases also linked to the effect of multiple vaccinations
. Notably, the adverse chronic phase of this syndrome affects 50-70% of flocks and up to 100% of animals within a flock. It is characterized by severe neurobehavioural outcomes (restlessness, generalized weakness, muscle tremors, loss of response to stimuli, ataxia, tetraplegia, stupor, coma and death), inflammatory lesions in the brain and the presence of aluminum in central nervous system tissues
. These latter findings thus confirm the ones by Khan et al.
who demonstrated the ability of aluminium adjuvants to penetrate the blood brain barrier, and further, they show that the resulting presence of aluminium in the brain can trigger severe neurological damage.
As a background, in 2008 a compulsory vaccination against bluetongue virus was implemented across Europe. In Spain, most sheep were subcutaneously vaccinated against two different viral serotypes and this represented four doses of vaccines in about a month with an estimated total amount of 16 mg of aluminum per animal. Shortly after (2-6 days), an acute neurological reaction was observed in a low but representative proportion of animals in a large number of vaccinated flocks across the whole country.
This “acute phase” was characterized by an array of acute severe nervous clinical signs such as lethargy, stupor, transient blindness, abnormal behavior and sometimes tremors at limbs and head and seizures in the most severely affected cases. Most animals apparently recovered from this phase and, between weeks and months later, an insidious and devastating wasting syndrome appeared in both, vaccinated flocks previously-affected by the acute phase or not.
This “chronic phase” was characterized by generalized weakness, muscle tremors and weight loss leading to extreme cachexia that could be followed by ataxia, tetraplegia and death. In certain geographical areas, spontaneous mortality in affected flocks increased a mean of 16.5% (range: 0.8%-65%; 26). Main lesions were severe meningoencephalitis in the acute phase and muscular atrophy, fat depletion and neurodegeneration in the chronic phase. Intensive investigations in this process were performed by many research groups and all known, compatible diseases of ovine were ruled out.
Remarkably, the chronic phase of the syndrome had been seen before compulsory vaccination against bluetongue virus by the authors in a small number of flocks. The sheep syndrome was reproduced in three lambs from a flock that had no previous history of vaccination. Over a period of 10 months, these animals were repetitively inoculated with aluminum-containing vaccines not only against bluetongue virus but also against other important ovine pathogens. In the whole experiment, the vaccinated lambs received a total amount 56 mg of aluminum divided into 14 inoculations. The clinical pictured observed was similar to the chronic phase in both the clinical and pathological aspect. Aluminum was found in a larger amount in nervous tissue of vaccinated animals
.
The weight of sheep at time of these inoculations was 45 kg, meaning that each sheep received 1.24 of aluminum/kg body weight. In Western countries, a typical child may be injected with as much as 4.225 mg of elemental Al by the age of 12 months
. Our review of currently licensed vaccine package inserts in the United States is consistent with this figure. For example, according to the standard U.S vaccination schedule, every vaccinated child receives a total of 5–6 mg of Al by the age of 2 years, or up to 1.475 mg of Al during a single visit to the pediatrician
. Given that vaccine-derived aluminum persist in the body and is absorbed at nearly 100% efficacy, this would mean that a 10 kg weight 12 month old baby would have an aluminum-adjuvant burden of 0.4225 mg/kg body weight which is approximately 3x less than the aluminum burden of the sheep reported in Lujan et al.’s
study.
This observation should give everyone a pause to think because it shows that the amounts of aluminum that produced the severe neurodegenerative ovine syndrome (which clearly is similar to some human diseases linked to the effect of multiple vaccinations) are in a range that is nearly comparable to human situation. In other words, Lujan’s sheep did not receive a “mammoth dose of aluminum” which would be clinically irrelevant.
Similarly to Lujan et al. my laboratory undertook detailed behavioural studies on new-born male and female mice given an “equivalent” to high and low exposure to aluminum from vaccines (according to the U.S. and Scandinavian vaccination schedules respectively)
. The results showed that aluminum injections in the neonatal period significantly increased anxiety-like behaviours and reduced exploratory activities in mice when they were tested as adults approximately 4 months later. These adverse behavioural outcomes were long-lasting and persisted throughout the two month period of testing
.
Later examinations by our lab have shown that the mice injected with the aluminum in the equivalent of what children in the U.S. receive via vaccinations have altered expression of certain genes in the brain.
Namely, pro-inflammatory genes were up-regulated, while a key neurotransmitter acethyl-cholinesterase (AChE) was down-regulated. Male mice were more affected. Just as males are more affected in autism.
Note that AChE has an anti-depression/anxiety effect. Low AChE activity is associated with deficits in neurodevelopment
.
In summary, aluminium salts are the most widely used adjuvants in current use. The fact that they can trigger pathological immunological responses and a cascade of adverse health effects is now well documented, albeit still not widely recognized in the medical community. The administration of continuously escalating doses of this poorly biodegradable adjuvant in the population should be far more carefully evaluated by regulatory agencies and the pharmaceutical industry than what has been the practice to date. It is likely that individual’s tolerance to aluminium may be compromised by a variety of factors including over-vaccination, blood-brain barrier immaturity, individual susceptibility factors (i.e., previous personal or familial history of autoimmune diseases), and aging that may be associated with both subtle blood-brain barrier alterations.
It is further likely that an increasing number of individuals, regardless of their genetic background, will react adversely if exposures to compounds with immune adjuvant properties exceed a certain threshold. This concept has in fact been clearly demonstrated by Tsumiyama et al.
who in 2009 showed that repeated immunization with antigen causes systemic autoimmunity in mice otherwise not prone to spontaneous autoimmune diseases.
It is true that people are exposed constantly to infectious agent in the environment, however, there is a vast difference between natural exposure and that induced by vaccinations. The reason for this is that the immune response induced by vaccination is greatly amplified, owing to the addition of adjuvants with immune-stimulating properties. This notion is further supported by the fact that vaccination produces a much higher and sustained level of antibodies compared to natural infection. For instance, Gardasil HPV vaccination induces a 40-fold increase in anti-HPV antibodies compared with the physiological antibody level triggered by a natural HPV infection
. The antibody titre against the HPV-16 and 18 may remain 11 times higher than those induced by a natural infection 5.5 years after vaccination
.
Similarly, CervarixTM has induced sustained antibody titres for HPV-18 more than 4-fold higher than natural infection titers at 8.4 years after initial vaccination with 100% seropositivity maintained
. It should also be noted that vaccinations are carried out almost exclusively for preventative measures and in the absence of an actual infection. In such a scenario, the vaccine-induced antibodies are more likely to preferentially bind to host antigens with which they share structural similarity. This phenomenon is well known under the term “molecular mimicry” and it has been clearly proven in the case of the antiphosholipid syndrome and the tetanus vaccine
.
Herd Immunity: Can Infectious Diseases be Prevented by High-Vaccination Coverage?
The frequent statement that high levels of vaccination prevent disease outbreaks is not accurate as infectious diseases do in fact occur even in fully vaccinated populations
as well as individuals
(see Table 1 for more examples). The likely reason for this is that vaccines primarily stimulate humoral immunity (antibody-based or Th2 responses) while they have little or no effect on cellular immunity (cytotoxic T-cells, Th1 responses), which is absolutely crucial for protection against viral as well as some bacterial pathogens
. This may be the reason why vaccine-induced immunities are transient, requiring booster shots, while naturally acquired immunity conferred by the cellular immune system in the absence of vaccination tends to be permanent. Taken together, these observations may explain why outbreaks of allegedly vaccine-preventable diseases do occur in fully vaccinated populations and why, immunity (or its absence) cannot be reliably determined on the basis of serologic determination (measure of antibody levels)
, which is the most common measure of vaccine efficacy in clinical trials
.
It should be noted that there is an instance where vaccinations could induce T-cell (Th1) responses and this is true in the case of repetitive immunizations with the same antigen (i.e., closely spaced “booster shoots”) however, the induction of such immune responses is deleterious as demonstrated by Tsumiyama et al.
who showed that CD4+ T cells from repeatedly-immunized mice acquire the ability to induce autoantibodies which results in autoimmune tissue injury akin to that seen in human autoimmune diseases.
As previously mentioned, from these experiments Tsumiyama et al.
concluded that systemic autoimmunity appears to be the inevitable consequence of over-stimulating the host’s immune ‘system’ by repeated immunization with antigen.
Table 1. Reports of infectious disease outbreaks despite high vaccination coverage.
Vaccine- or Hygiene-Preventable Diseases?
The prevalent view that vaccines are the sole cause of the disappearance of infectious diseases requires intellectual caution because it has been clearly demonstrated that factors such as clean water and improved sanitation, as well as better nutrition, availability of antibiotics, greater access to health care, and technological advances in maternal and neonatal medicine) have also played a major impact on infectious disease incidence
.
In fact, according to the U.S. Centers for Disease Control and Prevention (CDC), these measures accounted for 90% reduction in infant mortality and 99% reduction in maternal mortality since 1900
. So clearly then, vaccines could not have played a major role in health as often claimed. This fact (of major reduction in mortality rates due to better sanitation measures prior introduction of vaccines) is also illustrated by a 2002 review in Lancet Infectious Diseases
which clearly shows that the crude death rate from infectious diseases in the U.S. in the 20th century has decreased to baseline levels prior wide-spread introduction of vaccination practices (see Figure 1 below).
Figure 1. Source: Aiello and Larson
.
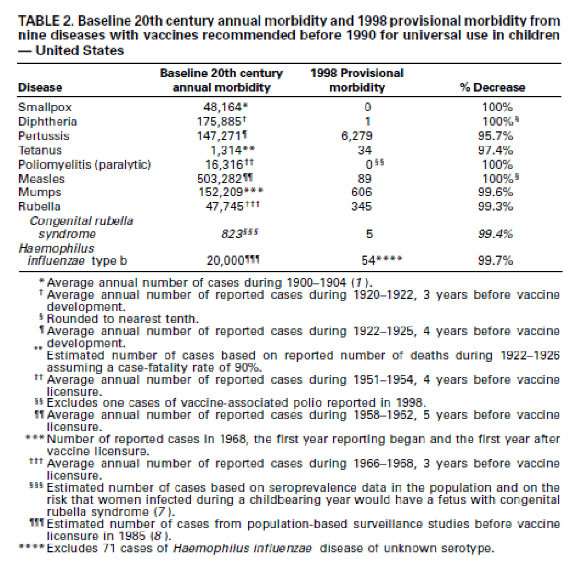
Remarkably when one tries to find solid research data in support of the claim that vaccines are responsible for historical eradication of diseases such as smallpox, polio etc, none is found. For example, the 1999 report from the U.S. CDC
(recently quoted by Kata
as proof that vaccines are responsible for the dramatic declines in morbidity and mortality from infectious diseases), titled “Ten Great Public Health Achievements -United States, 1900–1999”, lists the following Table:
This table only proves that the diseases listed decreased in incidence in the 20th century. It does not however prove that any of the vaccines were responsible for this decrease as there are other crucial factors which also changed during the course of the 20th century, such as improved hygiene, sanitation and nutrition. Remarkably, the U.S. CDC report lists these very factors (i.e., clean water and improved sanitation, as well as better nutrition, availability of antibiotics, greater access to health care, and technologic advances in maternal and neonatal medicine) among the top 10 achievements of the 20th century responsible for both control of infectious diseases and decreased infant mortality rates. Notably, these factors are listed separate from vaccines. Note also that like cholera and typhoid, polio is also a disease transmitted through contaminated water and is therefore a hygiene- reventable disease and not necessarily a vaccine-preventable disease. Altogether these observations invalidate the claim that infectious diseases such as polio would return should vaccination rates fall.
Benefits from Naturally- versus Vaccine-Acquired Immunity Scientific evidence has solidly established that naturally acquired childhood diseases provide long-term benefits to the immune system, including proper development of T-cell mediated immunity (which is crucial for long-term protection against infectious diseases), protection against asthma, allergies
, neurodegenerative diseases
and even protection against the most common and most aggressive type of primary brain tumors in humans (glioblastoma multiforme
). Unlike natural infections, vaccination can hamper the development of properly balanced T-cell mediated responses. For example, recent work shows that annual vaccination against influenza hampers the development of virus-specific CD8+T-cell immunity in children
.
SUMMARY
1) Many infectious diseases have been eradicated long before vaccines were introduced due to better sanitation, nutrition and vast improvements in medical care
2) Evidence of vaccine safety is very scant
3) Evidence of vaccine efficacy is dubious at best
4) Serious adverse reactions following routine vaccinations in children, including deaths, permanent neurological damage and disabling autoimmune and/or inflammatory conditions are known to occur even in individuals with no pre-existing conditions
Pushing of poorly tested drugs on children is neither acceptable nor ethical. Moreover, modern medical bioethics has rejected the notion that we can treat another individual(s) as a means to an end, regardless of how honourable that end may appear to be. The Nuremberg Code and subsequent Helsinki Declarations clearly reject the moral argument that the creation of alleged benefits for the many (“herd immunity”) justifies the sacrifice of the few. It should finally be noted that the proof of safety and efficacy of the product is the responsibility of regulatory agencies and drug producers and not the consumer. The former have been shown to be unreliable on many occasions due to financial interests
.
Dated: April 14, 2015
……………………………………………….
Lucija Tomljenovic, PhD
Neural Dynamics Research Group,
Dept. of Ophthalmology and Visual Sciences
University of British Columbia
828 W. 10th Ave, Vancouver, BC, V5Z 1L8
tel: 604-875-4111 (68375)
References
1. U.S. FDA, Workshop on Non-clinical Safety Evaluation of Preventative Vaccines: Recent Advances and
Regulatory Considerations. 2002. http://www.fda.gov/downloads/Biologi…/UCM054459.pdf
2. Poling, J.S., et al., Developmental regression and mitochondrial dysfunction in a child with autism. J Child
Neurol, 2006. 21(2): p. 170-2.
3. Yang, Y., et al., Acute metabolic crisis induced by vaccination in seven Chinese patients. Pediatr Neurol,
2006. 35(2): p. 114-8.
4. Ottaviani, G., A.M. Lavezzi, and L. Matturri, Sudden infant death syndrome (SIDS) shortly after
hexavalent vaccination: another pathology in suspected SIDS? Virchows Arch, 2006. 448(1): p. 100-4.
5. Aydin, H., E. Ozgul, and A.M. Agildere, Acute necrotizing encephalopathy secondary to diphtheria,tetanus
toxoid and whole-cell pertussis vaccination:diffusion-weighted imaging and proton MR spectroscopy
findings. Pediatr Radiol 2010. 40: p. 1281-1284.
6. Souayah, N., et al., Guillain-Barre syndrome after Gardasil vaccination: data from Vaccine Adverse Event
Reporting System 2006-2009. Vaccine, 2011. 29(5): p. 886-9.
7. Carvalho, J.F. and Y. Shoenfeld, Status epilepticus and lymphocytic pneumonitis following hepatitis B
vaccination. European J Int Med, 2008. 19: p. 383-385.
8. Zinka, B., et al., Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine,
2006. 24(31-32): p. 5779-80.
9. Mendoza Plasencia, Z., et al.,
vaccination against human papillomavirus]
. Neurologia, 2010. 25(1): p. 58-9.
10. Sutton, I., et al., CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis, 2009. 15(1): p.
116–119.
11. D’Errico, S., et al., Beta-tryptase and quantitative mast-cell increase in a sudden infant death following
hexavalent immunization. Forensic Sci Int, 2008. 179(2-3): p. e25-9.
12. Agmon-Levin, N., et al., Transverse myelitis and vaccines: a multi-analysis. Lupus, 2009. 18(13): p. 1198-
204.
13. Hofmann, C., M.O. Baur, and H. Schroten, Anti-NMDA receptor encephalitis after TdaP-IPV booster
vaccination: cause or coincidence? J Neurol, 2010. 258(3): p. 500-1.
14. DiMario, F.J., Jr., M. Hajjar, and T. Ciesielski, A 16-year-old girl with bilateral visual loss and left
hemiparesis following an immunization against human papilloma virus. J Child Neurol, 2010. 25(3): p.
321-7.
15. Karaali-Savrun, F., et al., Hepatitis B vaccine related-myelitis? Eur J Neurol, 2001. 8(6): p. 711-5.
16. Konstantinou, D., et al., Two episodes of leukoencephalitis associated with recombinant hepatitis B
vaccination in a single patient. Clin Infect Dis, 2001. 33(10): p. 1772-3.
17. U.S. FDA, FDA Science and Mission at Risk, Report of the Subcommittee on Science and Technology
2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-
4329b_02_01_FDA%20Report%20on%20Science%20and%20Technology.pdf
18. Zinka, B. and R. Penning, Unexplained cases of sudden infant death shortly after hexavalent vaccination.
Letter to Editor. Response to the comment by H.J. Schmitt et al. Vaccine 2006. 24: p. 5785–5786.
19. Tomljenovic, L. and C.A. Shaw, Mechanisms of aluminum adjuvant toxicity in pediatric populations.
Lupus, 2012. 21(2): p. 223-230.
20. Tomljenovic, L. and C.A. Shaw, One-size fits all? Vaccine, 2012. 30(12): p. 2040.
21. Tomljenovic, L. and C.A. Shaw, Human papillomavirus (HPV) vaccine policy and evidence-based
medicine: Are they at odds? Ann Med, 2013. 45(2): p. 182-93.
22. Tomljenovic, L. and C.A. Shaw, Aluminum Vaccine Adjuvants: Are they Safe? Current Medicinal
Chemistry, 2011. 18(17): p. 2630-2637.
23. Collignon, P., P. Doshi, and T. Jefferson, Adverse events following influenza vaccination in Australia–
should we be surprised? Rapid response to “Australia suspends seasonal flu vaccination of young
children”, BMJ 2010;340:c2419, http://www.bmj.com/content/340/bmj.c2419?tab=responses
24. Collignon, P., P. Doshi, and T. Jefferson, Australia suspends seasonal flu vaccination of young children.
BMJ, 2010. 340:c2419. http://www.bmj.com/content/340/bmj.c2419
25. Fierce Vaccines. Lawsuits claiming Merck lied about mumps vaccine efficacy headed to trial.
http://www.fiercevaccines.com/story/lawsuits-claiming-merck-lied-about-mumps-vaccine-efficacy-headed-
trial/2014-09-09
26. Demicheli, V., et al., Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev,
2005(4): p. CD004407.
27. Fombonne, E. and S. Chakrabarti, No evidence for a new variant of measles-mumps-rubella-induced
autism. Pediatrics, 2001. 108(4): p. E58.
28. Poland, G.A., I.G. Ovsyannikova, and R.M. Jacobson, Vaccine immunogenetics: bedside to bench to
population. Vaccine, 2008. 26(49): p. 6183-8.
29. Poland, G.A. and R.M. Jacobson, The age-old struggle against the antivaccinationists. N Engl J Med,
2011. 364(2): p. 97-9.
30. Kovel, A., et al., Safety and immunogenicity of acellular diphtheria-tetanus-pertussis and Haemophilus
conjugate vaccines given in combination or at separate injection sites. J Pediatr, 1992. 120(1): p. 84-7.
31. Kaplan, S.L., et al., Immunogenicity and safety of Haemophilus influenzae type b-tetanus protein conjugate
vaccine alone or mixed with diphtheria-tetanus-pertussis vaccine in infants. J Pediatr, 1994. 124(2): p. 323-
7.
32. Li, G., et al., Safety and immunogenicity of a diphtheria, tetanus, acellular pertussis and Haemophilus
influenzae Type b combination vaccine compared with separate administration of licensed equivalent
vaccines in Chinese infants and toddlers for primary and booster immunization. Vaccine, 2010. 28(25): p.
4215-23.
33. Shinefield, H., et al., Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in
healthy children. Pediatr Infect Dis J, 2005. 24(8): p. 665-9.
34. Velu, V., et al., Comparative efficacy of two dosages of recombinant hepatitis B vaccine in healthy
adolescents in India. Pediatr Infect Dis J, 2007. 26(11): p. 1038-41.
35. Thomas, C. and M. Moridani, Interindividual variations in the efficacy and toxicity of vaccines.
Toxicology, 2009. 278(2): p. 204-10.
36. World Health Organization (WHO). Weekly and epidemiological record. 7 Jan 2005,
http://www.who.int/wer/2005/wer8001.pdf
37. Petrik, M.S., et al., Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice.
Neuromolecular Med, 2007. 9(1): p. 83-100.
38. Shaw, C.A. and M.S. Petrik, Aluminum hydroxide injections lead to motor deficits and motor neuron
degeneration. J Inorg Biochem, 2009. 103(11): p. 1555-62.
39. Li, X., et al., Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat
brains. Nanomedicine, 2009. 5(4): p. 473-479.
40. Passeri, E., et al., Long-term follow-up of cognitive dysfunction in patients with aluminum hydroxide-
induced macrophagic myofasciitis (MMF). J Inorg Biochem, 2011. 105(11): p. 1457-63.
41. Couette, M., et al., Long-term persistence of vaccine-derived aluminum hydroxide is associated with
chronic cognitive dysfunction. J Inorg Biochem, 2009. 103(11): p. 1571-8.
42. Khan, Z., et al., Slow CCL2-dependent translocation of biopersistent particles from muscle to brain. BMC
Med, 2013. 11: p. 99.
43. Shaw, C.A. and L. Tomljenovic, Aluminum in the central nervous system (CNS): toxicity in humans and
animals, vaccine adjuvants, and autoimmunity. Immunol Res, 2013. 56(2-3): p. 304-316.
44. Shaw, C.A., et al., Etiology of autism spectrum disorders: Genes, environment, or both? OA Autism, 2014.
2(2): p. 11.
45. Shaw, C.A., Y. Li, and L. Tomljenovic, Administration of aluminium in vaccine-relevant amounts in
neonatal mice is associated with long-term adverse neurological outcomes. J Inorg Biochem, 2013. 128: p.
237-44.
46. Shaw, C.A., D. Li, and L. Tomljenovic, Are there negative CNS impacts of aluminum adjuvants used in
vaccines and immunotherapy? Immunotherapy, 2014. 6(10): p. 1055-71.
47. Cadusseau, J., et al., Selective elevation of circulating CCL2/MCP1 levels in patients with longstanding
post-vaccinal macrophagic myofasciitis and ASIA. Curr Med Chem, 2014. 21(4): p. 511-7.
48. Offit, P.A. and R.K. Jew, Addressing parents’ concerns: do vaccines contain harmful preservatives,
adjuvants, additives, or residuals? Pediatrics, 2003. 112(6 Pt 1): p. 1394-7.
49. Eldred, B.E., et al., Vaccine components and constituents: responding to consumer concerns. Med J Aust,
2006. 184(4): p. 170-5.
50. Yokel, R.A., C.L. Hicks, and R.L. Florence, Aluminum bioavailability from basic sodium aluminum
phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem Toxicol,
2008. 46(6): p. 2261-6.
51. Yokel, R.A. and P.J. McNamara, Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol,
2001. 88(4): p. 159-67.
52. Gherardi, R.K., et al., Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived
aluminium hydroxide in muscle. Brain, 2001. 124(Pt 9): p. 1821-31.
53. Lujan, L., et al., Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in
commercial sheep. Immunol Res, 2013. 56(2-3): p. 317-324.
54. Mitkus, R.J., et al., Updated aluminum pharmacokinetics following infant exposures through diet and
vaccination. Vaccine, 2011. 29(51): p. 9538-43.
55. Tomljenovic, L., Aluminum and Alzheimer’s Disease: After a Century of Controversy, Is there a Plausible
Link? Journal of Alzheimers Disease, 2011. 23(4): p. 567-598.
56. Suzuki, K., et al., Reduced acetylcholinesterase activity in the fusiform gyrus in adults with autism
spectrum disorders. Arch Gen Psychiatry, 2011. 68(3): p. 306-13.
57. Tsumiyama, K., Y. Miyazaki, and S. Shiozawa, Self-organized criticality theory of autoimmunity. PLoS
One, 2009. 4(12): p. e8382.
58. Harro, C.D., et al., Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1
virus-like particle vaccine. J Natl Cancer Inst, 2001. 93(4): p. 284-92.
59. Bayas, J.M., L. Costas, and A. Munoz, Cervical cancer vaccination indications, efficacy, and side effects.
Gynecol Oncol, 2008. 110(3 Suppl 2): p. S11-4.
60. Harper, D.M. and K.B. Williams, Prophylactic HPV vaccines: current knowledge of impact on gynecologic
premalignancies. Discov Med, 2010. 10(50): p. 7-17.
61. Dimitrijevic, L., et al., Vaccine model of antiphospholipid syndrome induced by tetanus vaccine. Lupus,
2012. 21(2): p. 195-202.
62. Blank, M., et al., Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious
etiology of antiphospholipid syndrome. J Clin Invest, 2002. 109(6): p. 797-804.
63. Centers for Disease Control and Prevention (CDC), Diphtheria outbreak–Russian Federation, 1990-1993.
MMWR Morb Mortal Wkly Rep, 1993. 42(43): p. 840-1, 847.
64. Kim, H.W., et al., Respiratory syncytial virus disease in infants despite prior administration of antigenic
inactivated vaccine. Am J Epidemiol, 1969. 89(4): p. 422-34.
65. Romagnani, S., Biology of human TH1 and TH2 cells. J Clin Immunol, 1995. 15(3): p. 121-9.
66. Zweerink, H.J. and L.W. Stanton, Immune response to herpes simplex virus infections: virus-specific
antibodies in sera from patients with recurrent facial infections. Infect Immun, 1981. 31(2): p. 624-30.
67. Miller, E., et al., Safety and immunogenicity of co-administering a combined meningococcal serogroup C
and Haemophilus influenzae type b conjugate vaccine with 7-valent pneumococcal conjugate vaccine and
measles, mumps and rubella vaccine at 12 months of age. Clin Vaccine Immunol, 2011. 18(3): p. 367–372.
68. Centers for Disease Control and Prevention (CDC), Measles outbreak among vaccinated high school
students–Illinois. Morb Mortal Wkly Rep (MMWR) 1984 Jun 22;33(24):349-51,
http://www.cdc.gov/mmwr/preview/mmwrhtml/00000359.htm
69. Anderson, L.J. and J.F. Seward, Mumps epidemiology and immunity: the anatomy of a modern epidemic.
Pediatr Infect Dis J, 2008. 27(10 Suppl): p. S75-9.
70. Boxall, N., et al., An increase in the number of mumps cases in the Czech Republic, 2005-2006. Euro
Surveill, 2008. 13(16).
71. Sutter, R.W., et al., Outbreak of paralytic poliomyelitis in Oman: evidence for widespread transmission
among fully vaccinated children. Lancet, 1991. 338(8769): p. 715-20.
72. Pedersen, I.R., et al., Subclinical measles infection in vaccinated seropositive individuals in arctic
Greenland. Vaccine, 1989. 7(4): p. 345-8.
73. Ozanne, G. and M.A. d’Halewyn, Secondary immune response in a vaccinated population during a large
measles epidemic. J Clin Microbiol, 1992. 30(7): p. 1778-82.
74. Internal Medicine News. 22 Nov 2011. Infectious Diseases Society of America Conference. Acellular
Pertussis Vaccine’s Waning Immunity Caused California Epidemic. http://www.pediatricnews.com/single-
article/acellular-pertussis-vaccine-s-waning-immunity-caused-california-
epidemic/71de9826f4686b12b3fe2bc85984044a.html
75. Aiello, A.E. and E.L. Larson, What is the evidence for a causal link between hygiene and infections?
Lancet Infect Dis, 2002. 2(2): p. 103-10.
76. Centers for Disease Control and Prevention (CDC), Ten great public health achievements–United States,
1900-1999. MMWR Morb Mortal Wkly Rep, 1999. 48(12): p. 241-3.
77. Kata, A., Anti-vaccine activists, Web 2.0, and the postmodern paradigm – An overview of tactics and tropes
used online by the anti-vaccination movement. Vaccine, 2011.
78. Silverberg, J.I., et al., Association between varicella zoster virus infection and atopic dermatitis in early
and late childhood: a case-control study. J Allergy Clin Immunol, 2010. 126(2): p. 300-5.
79. Silverberg, J.I., et al., Varicella zoster virus (wild-type) infection, but not varicella vaccine, in late
childhood is associated with delayed asthma onset, milder symptoms, and decreased atopy. Pediatr Asthma
Allergy Immunol 2009. 22: p. 15-20.
80. Illi, S., et al., Early childhood infectious diseases and the development of asthma up to school age: a birth
cohort study. BMJ, 2001. 322(7283): p. 390-5.
81. Sasco, A.J. and R.S. Paffenbarger, Jr., Measles infection and Parkinson’s disease. Am J Epidemiol, 1985.
122(6): p. 1017-31.
82. Wrensch, M., et al., History of chickenpox and shingles and prevalence of antibodies to varicella-zoster
virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol, 2005.
161(10): p. 929-38.
83. Bodewes, R., et al., Annual vaccination against influenza virus hampers development of virus-specific CD8
T cell immunity in children. J Virol, 2011. 85(22): p. 11995-2000.
84. DeAngelis, C.D. and P.B. Fontanarosa, Impugning the integrity of medical science: the adverse effects of
industry influence. JAMA, 2008. 299(15): p. 1833-5.
85. Angell, M., Industry-sponsored clinical research: a broken system. JAMA, 2008. 300(9): p. 1069-71.
86. Lenzer, J., Whistleblower charges medical oversight bureau with corruption. BMJ, 2004. 329(7457): p. 69.
87. Lenzer, J., Cochrane Collaboration’s stand versus industry funding. CMAJ, 2004. 171(2): p. 122.
88. Lenzer, J., FDA’s counsel accused of being too close to drug industry. BMJ, 2004. 329(7459): p. 189.
89. Lenzer, J., Scandals have eroded US public’s confidence in drug industry. BMJ, 2004. 329(7460): p. 247.
90. Lenzer, J., Crisis deepens at the US Food and Drug Administration. BMJ, 2004. 329(7478): p. 1308.
91. Wager, E., Authors, ghosts, damned lies, and statisticians. PLoS Med, 2007. 4(1): p. e34.
92. Rising, K., P. Bacchetti, and L. Bero, Reporting bias in drug trials submitted to the Food and Drug
Administration: review of publication and presentation. PLoS Med, 2008. 5(11): p. e217; discussion e217.
93. Berger, E., Ghostwriters, data manipulation and dollar diplomacy: how drug companies pull the strings in
clinical research. Ann Emerg Med, 2008. 52(2): p. 137-9.
94. Rothman, S.M. and D.J. Rothman, Marketing HPV vaccine: implications for adolescent health and medical
professionalism. JAMA, 2009. 302(7): p. 781-6.
95. Tomljenovic, L. and C.A. Shaw, Too Fast or Not Too Fast: The FDA’s Approval of Merck’s HPV Vaccine
Gardasil. J Law Med Ethics, 2012. 40(3): p. 673-81.
96. Jefferson, T., Influenza vaccination: policy versus evidence. BMJ, 2006. 333(7574): p. 912-5.
97. Collignon, P., P. Doshi, and T. Jefferson, Child influenza vaccination. Ramifications of adverse events in
children in Australia. BMJ, 2010. 340: p. c2994.
Featured Image Contributing Source: Pixabay / PublicDomainPictures
Want to continue reading?
Enter your details below to read more and receive updates via email.